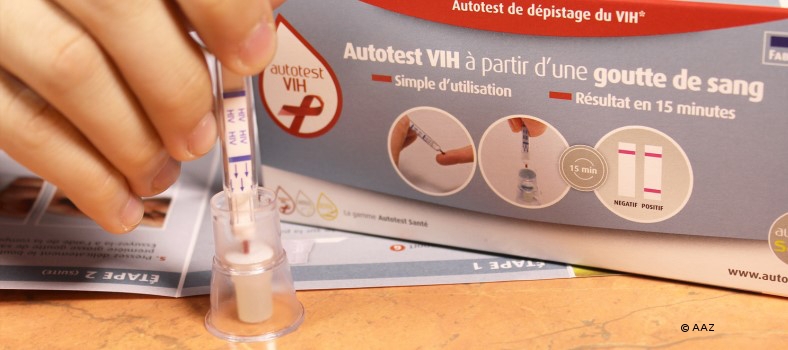
The first HIV test for home use was approved in the USA in July 2012. Since the end of April this year, a test kit with CE labelling for laypeople has been available in pharmacies in the UK. According to the French Minister of Health, a home test is to be launched on the market this year. And what is happening in Germany?
Home tests are intended to reach people who avoid a test in the healthcare system because they fear being stigmatised by an HIV infection. It is also hoped that people at higher risk will take an HIV test more frequently with the help of this service. However, this has not yet been proven. In the USA, an average of around 25 % pharmacies were offering home tests one year after their introduction. The test kits were often only available over the counter - so the fear of stigmatisation continues into the pharmacies.
The test from the USA costs around 40 dollars, while a price of 25 euros is planned for the home test in France. At these prices, it is questionable whether people who have not yet been reached by testing projects and the healthcare system will actually take the test for home use. As the home tests still have the difficulty of lower sensitivity (accuracy) - especially the tests with oral fluid - home test users get poorer quality for a lot of money than with a test in the healthcare system. In addition, there is no counselling and it remains unclear whether and to what extent a positive test result will result in access to treatment.
In Germany, the sale of HIV tests has been fundamentally restricted since 2009 by §11 (3a) of the Medical Devices Act: Laypersons cannot purchase HIV tests. Following the approval of a home test with CE labelling in the UK, it is to be expected that the EU Commission will demand an amendment to the Medical Devices Act with a view to the free movement of goods within the EU and that HIV home tests will also be available here.
HIV submission tests - the alternative?
In contrast to home tests, HIV home collection tests only require the user to collect the sample (oral fluid or blood from the fingertip). The sample tube is then sent to the laboratory where the results are determined using conventional tests. The result is then communicated - usually by telephone. Single-sender tests are a low-threshold option, especially for people who regularly need an HIV test.
Initial experience with HIV submission tests in Europe was gained in a pilot project in the UK from January to November 2013: almost 10,000 people requested a test kit, which was sent by post. 64 % sent in a sample, of which 73 % were MSM (men who have sex with men). Result: 105 samples tested HIV-positive, which corresponds to 1.7 % (MSM 1.8 %). Negative test results were communicated by text message, reactive HIV tests by telephone call. This ensured that counselling and referral for confirmatory diagnostics could take place. A third of the clients had never been tested before. The method was therefore used to acquire "new customers". The proportion of positive samples of 1.7 % is also high compared to positive tests in hospitals and test projects.
An EU project is currently being launched to trial HIV submission tests for MSM: EURO HIV EDAT. The sample material is a swab from the oral mucosa. In addition to Spain, Belgium, Denmark, Portugal and Romania, Germany is also represented in this test project by Aidshilfe Nordrhein-Westfalen.
Risks of HIV submission tests and legal situation
Misuse in the sense of (secretly) testing other people is generally possible with single-use tests - however, this risk can be minimised if the test tubes are only given after counselling and information. In contrast to the home test, there is the possibility of (telephone) counselling and the involvement of the HIV-positive person tested in further diagnostics or therapy.
In contrast to HIV home tests in Germany, single-sender tests pose a greater challenge in terms of medical law: As soon as people other than the test user are involved, legal regulations come into play: According to §24 of the Infection Protection Act, only doctors are permitted to treat patients with communicable diseases. Treatment" also includes the "direct and indirect detection of a pathogen for the determination of an infection or communicable disease" - i.e. diagnostics.
Based on the opinion of the Imperial Court of Justice in 1894 and the established case law of the Federal Court of Justice, a medical intervention - including diagnostics - without consent constitutes bodily harm. Written consent alone - as in the UK - is not sufficient. A legally unproblematic solution would be to inform clients personally in test projects and then provide them with a few submission tests for the coming months or years.
The text is an abridged version. The more extensive and detailed version on the topic by DAH medical officer Armin Schafberger can be found in the magazine.hiv of Deutsche AIDS-Hilfe: http://blog.aidshilfe.de/2015/08/15/hiv-tests-fuer-zu-hause/